The FDA has issued several warning letters to firms that market supplements that allegedly contain cannabidiol (CBD). As part of these actions, FDA has tested the chemical content of cannabinoid compounds in some of the leading products, and many were found to not contain the levels of CBD they claimed.
Many were "impure" in that they contained a higher amount of the hallucinogenic THC than claimed.
In a recent Dutch study of CBD content and purity, 24 of the 26 leading products analyzed had misleading claims.
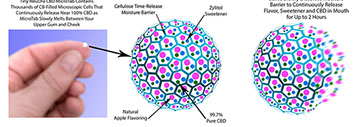
In contrast NeuOra's claim of 99.7% pure CBD have been certified by leading laboratories and government agencies.